Remidio AI detects diabetic retinopathy on smartphone
- March 7, 2023
- Steve Rogerson
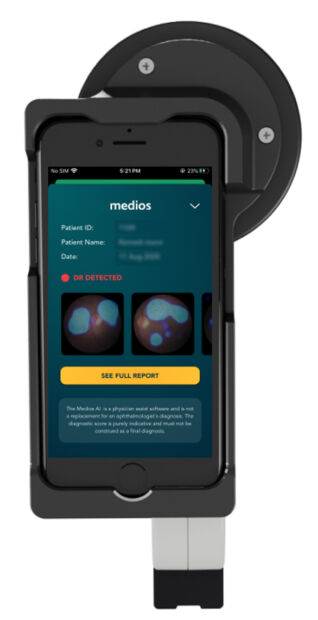
Indian company Remidio has received CE mark approval for its use of artificial intelligence (AI) on a smartphone to detect diabetic retinopathy (DR), a complication of diabetes that can cause vision loss and even blindness.
Its Medios AI can detect referable DR. The EU MDR Class II regulatory approval closely follows the nod the AI received from Singapore’s Health Sciences Authority (HSA).
With an estimated 537 million adults living with diabetes today, DR is predicted to be a leading cause of blindness globally.
Divya Rao, Remidio’s medical director, called the approval a “key milestone” as the first step towards transforming global DR screening.
Medios AI is a smartphone-based automated algorithm that works offline. The algorithm harnesses deep-learning technology deployed on a smartphone-based fundus camera, Remidio NM-FOP, to detect referable DR within ten seconds.
Sobha Sivaprasad, professor and consultant ophthalmologist at Moorfields Eye Hospital in London, said: “Given the growing burden of diabetes and the challenge of establishing and maintaining national DR screening programmes, this AI integrated on a lightweight camera can be utilised in any primary care clinic managing diabetes as it requires minimal expertise, leading to greater coverage of DR screening.”
The portable nature combined with no internet for inferencing ensures unhindered eye care delivered closer to the patient. Such intuitive technology can propel physicians to the forefront of closing the care gap for DR, the first point-of-contact for patients.
Rao added: “This enables newer models of screening by non-eye care professionals that are efficient and bring convenience without bulky equipment.”
Remidio’s vision to eradicate preventable blindness has led it to publish research on a device-agnostic application for Medios DR AI. The algorithm can now be used on desktop and smartphone-based fundus cameras. With additional AI under development to screen for retinal and systemic conditions, the company believes the scope of NM-FOP is limitless.
Medios AI is not FDA-approved and not available for sale in the USA.
Remidio is a Bengaluru-based ISO-13485-certified medical technology company that seeks to impact preventable blindness by creating accessible technologies that are smart and simple to use. Its CE-marked and FDA 510k-registered devices have helped screen and impact more than ten million patients globally.




