Telli FDA approved 4G SpO2 device for Covid
- January 3, 2022
- William Payne
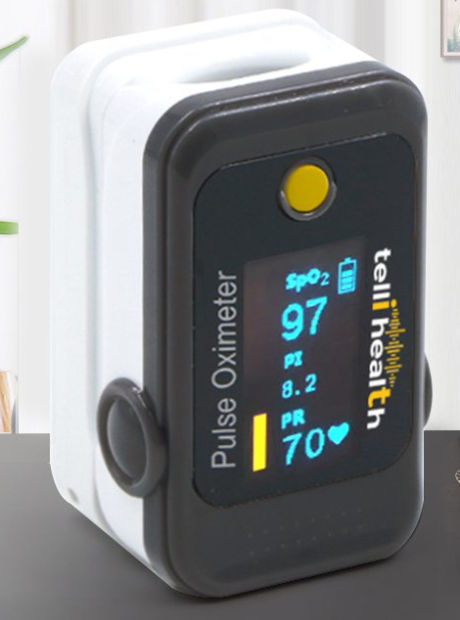
Telli Health has launched what it describes as the first FDA certified and approved 4G cellular connected SpO2 oximeter. The new device addresses the need for remote SpO2 patient monitoring devices (RPM) to monitor COVID-19 patients.
The pulse oximeter expands Telli Health’s 4G range of remote physiological health monitoring devices. The range also includes a touchless thermometer, blood pressure monitor, blood glucometer, and weight scale, and serve as a COVID-19 kit to monitor pre- and post-discharged patients without additional equipment required. Patient data is automatically transmitted directly into their existing health record and are available to their provider.
Telli Health devices use SIM technology that connects to all major carriers including Verizon, AT&T, T-Mobile/ Sprint, providing a solution to patients in the most rural and remote areas, improving accessibility and allowing more patients to be reached.
Telli Health Director of Product Support Alvaro Salgado, said, “The pulse oximeter is the main device used to monitor COVID patients, so I am thrilled that our 4G cellular-connected SpO2 pulse oximeter is now being delivered to providers – Telli Health is responding to that demand. Providers have reported in the past that COVID patients indicated that they were feeling fine, when oxygen saturation levels were quite low. Our cellular-connected SpO2 pulse oximeter will alert providers of a problem and give them ample time to act accordingly. Our pulse oximeter is also significantly advantageous for its ease of use and low power requirements. This revolutionary technology will allow patients to remain at their hospital-in-home to recover in a familiar place in lieu of a traditional hospital room.”




